Although highly standardised programmes of Operational Excellence (OPEX) have been implemented in almost every globally operating pharmaceutical company, the success of OPEX initiatives differs considerably. This article presents, based on the St.Gallen OPEX understanding, an overview of the factors that enable a sustainable OPEX culture and OPEX implementation.
Over the past decade, the importance of Operational Excellence (OPEX) in the pharmaceutical industry has grown significantly. A mere copy and paste from successful automotive excellence programmes does not work for the pharmaceutical structural requirements in the long run (Friedli et al., 2013). This has been realised by most of the pharma companies in the past ten years while working their way with individual roadmaps. Accordingly Lean, along with Six Sigma has grown in prominence with their principles, methodologies and tools supporting OPEX initiatives (Friedli et al., 2010, p.220). However, the evolution of OPEX in the pharma industry showed that the pathway to OPEX is more than just about applying tools. As each OPEX initiative is shaped by a company’s culture, they tend to vary to a large extent with no universal recipe. But based on the research and project experiences of the Institute of Technology Management of the University of St.Gallen, Switzerland (ITEM-HSG) some common guidelines and procedures are identified.
 Modern approaches to OPEX have evolved from the understanding of Lean Production and are generally regarded as part of continuous, corporate improvement concepts (Friedli & Schuh, 2012). However, OPEX programmes cannot be viewed as standalone or as a set of new methods and tools as they comprise and rely on several already established manufacturing concepts (Friedli et al., 2010). Operational excellence is about the continuous pursuit of improvement of a production plant in all dimensions. Improvement is measured by balanced performance metrics comprising efficiency and effectiveness, thus providing a correlative basis for improvement evaluation (Friedli et al. 2013, p.24).
Modern approaches to OPEX have evolved from the understanding of Lean Production and are generally regarded as part of continuous, corporate improvement concepts (Friedli & Schuh, 2012). However, OPEX programmes cannot be viewed as standalone or as a set of new methods and tools as they comprise and rely on several already established manufacturing concepts (Friedli et al., 2010). Operational excellence is about the continuous pursuit of improvement of a production plant in all dimensions. Improvement is measured by balanced performance metrics comprising efficiency and effectiveness, thus providing a correlative basis for improvement evaluation (Friedli et al. 2013, p.24).
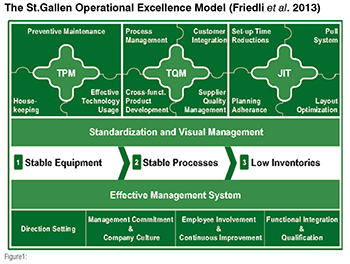
The philosophy of OPEX can be traced back to research results on excellence by Drucker (1971), Peters and Waterman (1982), Hayes and Wheelwright (1985), and Schonberger (1986), complemented by a long history of Japanese manufacturing concepts strongly linked to the Toyota Production System, which was first published by Sugimori (1977). In 2004, the ITEM-HSG started its activities in the field of pharmaceutical manufacturing to better understand OPEX and its implementation level in the industry. Based on the work of Cua et al. (2001) the ITEM-HSG developed a framework for the structured discussion of OPEX in a pharmaceutical context – the so called St.Gallen Operational Excellence Model (see Figure1) was established.
On the highest level of abstraction, this model can be divided into two larger sub-systems: a technical and a social sub-system.
The technical sub-system comprises of Lean practices like Total Productive Maintenance (TPM), Total Quality Management (TQM), and Just-in-Time (JIT). Most of these major operations management principles usually aim at a certain area of concern (such as low equipment availability, low quality, high inventories); companies implement them in order to address exactly these issues. Based on the first benchmarking results the ITEM-HSG structured these three sub-elements in a logical sequence in their implementation, namely: first TPM, second TQM and third JIT. Without TPM, the goals of TQM cannot be achieved, as there can be no stable process based on unstable equipment. The mastering of TPM and TQM are prerequisites to be able to take out waste without facing the danger that the whole underlying system starts to crash. (Friedli et al., 2013, p.17)
Second, there is a ‘social’ sub-system, the so called Effective Management System (EMS) which takes up the quest for an operational characterisation of management quality and work organisation. This second system focuses on supporting and encouraging people to continuously improve processes. (Friedli et al., 2013, p.20)
Standardisation and visual management cannot be clearly related to either TPM, TQM or JIT. We call them basic elements because they can be regarded as basic prerequisites for successfully implementing the whole technical sub-system in operations and administration. As Imai (1986) explained in his book on continuous improvement, it is impossible to improve any process before it has been standardised, and thus stabilised. Visual management provides the workforce with updated information on process and performance data which assists the deployment of TPM, TQM, and JIT principles. (Friedli et al., 2013, p.20)
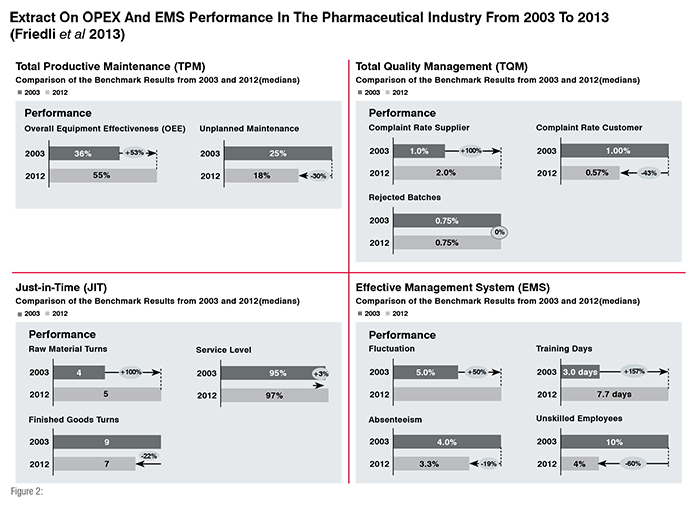
The St.Gallen OPEX benchmarking assesses a set of production-specific KPIs that are closely linked to the technical sub-system (comprising TPM, TQM and JIT), as well as the social sub-system; Totally 50 operational KPIs are collected and analysed. A look at the results of the past 10 years shows an improvement in performance of the global pharmaceutical industry in terms of both effectiveness and efficiency (Friedli et al. 2013). Figure 2 indicates an extract of the benchmarking. From a TPM perspective the major advancement is the increased awareness of the relationship between good maintenance and good quality. In the TQM section, a positive development of the Complaint Rate Customer can be shown. It has decreased from 1 per cent in 2003 to 0.57 per cent in 2012. The Rejected Batches score (given as percentage of all batches produced) stayed at 0.75 per cent from 2003 to 2012. Looking at JIT performance, the median score reveals a change in Raw Material Turns i.e., from 4 turns per year in 2003 to 5.35 turns per year in 2012. Companies are increasingly trying to deliver a demand-oriented JIT instead of a stock-oriented approach. In the St.Gallen OPEX benchmarking (EMS sub-system) absenteeism and fluctuation are used as measures of employee satisfaction. Absenteeism, measured as the percentage of the total working time an employee is absent, decreased from 4 per cent in 2003 to 3.3 per cent in 2012. Fluctuation, however, increased by about 50 per cent from 5 per cent in 2003 to 7.5 per cent in 2012. (Friedli et al., 2013)
The success of an OPEX program depends, to a greater extent on leadership and behavioural skills than on technical skills (Friedli et al., 2010, p.202). Managers often think ‘Changing Culture’ leads to ‘Changes in the Work’, but in fact it is backwards and culture is more an outcome than an input. ‘Changing the Work’ leads to ‘Change in the Culture’ as OPEX is a new way of leading, new way of working, and new way of thinking. Schein (1985) defines organisational culture as a set of artefacts (visible behaviour), values (rules, standards), and assumptions (invisible, unconscious) that are shared by members of an organisation. Creating and sustaining an organisational OPEX culture is a key challenge for the leadership team. Leadership characterised by a participative leadership style is essential to establish a high level of collaboration. Continuous improvement, the main philosophy of OPEX programmes requires shared tasks, empowerment, and teamwork with clear rules. Thereby it needs to be ensured that decisions are made at the lowest possible organisational level. Thus, the more individuals become involved in the decision-making process, the more variety and more ideas will be created. Further initiating a cultural change is mainly driven by activities designed and coordinated from corporate level. During the first stage of implementation, corporate support is absolutely essential. An obtrusive communication of the benefits and need for continuous improvement inside the organisation is key aspect for the OPEX leader. Besides this, the execution of activities with a visible benefit and sense for employees as well as the credible behaviour is mandatory to successfully and sustainably manage OPEX.
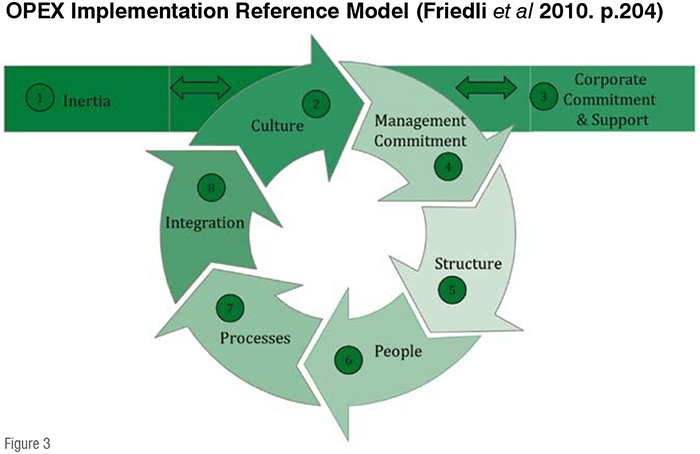
Underlying values have a strong influence on the behaviour of employees, as values define how they behave, regardless of the situation and context (Modig & Ahlström, 2012). Learning experiences of the organisational members, especially those at site level, influence these values and consequently the sustainable success of OPEX. Besides corporate commitment, it is also essential to gain the site management‘s commitment for the deployment of OPEX in order to sustain cultural change at site level. This commitment should go beyond formal agreements and include the active involvement of the site leadership (Friedli et al., 2010, p.203). A common approach designed at a corporate level needs to be tailored for each site’s specific needs to a certain level. In a research project with a leading pharma company, the St.Gallen OPEX team developed an ‘OPEX Implementation Reference Model’ which comprises eight categories of influencing institutional and process-related factors (Figure 3). In each of these subcategories, practices were identified that supported or hampered a sustainable implementation. (Friedli et al., 2010, p.205)
The category of ‘Organisational Inertia‘ describes the degree to which a site is capable of adopting new practices and initiatives, i.e. of changing current or past practices and ways of working and thinking. An organisation‘s ‘culture‘ is the sum of its past and current assumptions, experiences, philosophy and values, and is expressed in its self-image, inner workings, interactions with its stakeholders and future expectations. This addresses differences in the availability of highly professionalised corporate support, the connection between site objectives as defined in the vision and mission statement and OPEX objectives, as well as the visible engagement of corporate support people. ‘Management commitment’ means that the high level executives at site level directly participate in and pay attention to OPEX activities. The category of ‘organisational structure’ deals with the organisational integration of OPEX and available resources. The category of ‘people’ describes the level of general understanding of OPEX across the organisation as well as engagement and training of shop floor employees. The category of ‘implementation process’ focuses on the degree of standardisation in dealing with OPEX projects from idea selection to knowledge management. The more an initiative becomes a regular part of organisational activities the more standardisation can streamline activities. ‘Integration’ describes the process of attaining close and seamless coordination between departments, groups, systems and other corporate initiatives. (Friedli et al. 2013, p.206ff)
Enabling factorsDuring the research and project work of the ITEM-HSG OPEX team over the past ten years it has become clear that patterns of OPEX
Summary
Today most pharmaceutical manufacturers apply selected approaches, principles and methods as well as tools from OPEX in order to increase efficiency. Operational Excellence (OPEX) as a continuous pursuit of improvements in all dimensions leads to changes in existing working environments. Changes in the work environment in the long term lead to changes in the culture. A culture of Operational Excellence is not a bunch of written rules by the management team; it is the decision by the organisation to commit to go beyond the ordinariness and not being satisfied with the current status-quo. The major driver on a cultural level is the promotion of OPEX with all its aspects and the increased effort in training. As each OPEX initiative is shaped by an individual company’s culture, OPEX initiatives can vary to a large extent and it is the task of the OPEX leaders to balance the initiative (Friedli et al., 2013, p.114). An organisation with an OPEX culture provides personal and professional satisfaction for the employees about what they do and gives the company some kind of legitimisation and purpose, thereby motivating its members to make a contribution in view of achieving superior goals (Friedli et al., 2010, p.206).
Literature
Cua K. O., McKone K. E., Schroeder R. G. (2001). Relationships between implementation of TQM, JIT, and TPM and manufacturing performance. Journal of Operations Management, Vol. 19(2), pp. 675–694.
Drucker P.F. (1971). What we can learn from Japanese management. Harvard Business Review, Vol. 49 No. 2, pp. 110-22.
Friedli T., Basu P.B., Gronauer T., Werani J. (2010). The pathway to operational excellence in the pharmaceutical industry - Overcoming the internal criteria. Editio Cantor Verlag, Aulendorf.
Friedli T. Basu P.B., Bellm D.,Werani J. (2013). Leading pharmaceutical operational excellence - Outstanding practices and cases. Springer,Berlin, Heidelberg.
Friedli, T., Schuh, G. (2012). Wettbewerbsfähigkeit der Produktion an Hochlohnstandorten. 2. Auflage, Springer Verlag, Berlin, Heidelberg.
Hayes R.H., Wheelwright S.C., (1985). Restoring Our Competitive Edge: Competing Through Manufacturing. Wiley, New York.
Imai M. (1986) Kaizen: The Key to Japan's Competitive Success. Random House, New York.
Modig N., Ahlström P. (2012). This is lean. Resolving the efficiency paradox. Rheologica Publishing, Stockholm.
Peters, T.J. and Waterman, R.H. (1982), In Search of Excellence – Lessons from America’s Best-Run Companies, HarperCollins Publishers, London.
Schein, E. H. (1985). Organizational culture and leadership: A dynamic view. Jossey-Bass, San Francisco, CA.
Schonberger R.J., (1986). Japanese Manufacturing Techniques. The Free Press, New York.
Sugimori Y., Kusunoki K., Cho F., Uchikawa S. (1977). Toyota Production System and Kanban system: materialization of just-in-time and respect-for-human system. International Journal of Production Research, Vol. 15 (6), pp. 553–564.
-- Issue 21 --