Contamination remains one of the major dangers to the integrity of pharmaceutical products. The risks associated with contamination are greatly increased through the current trends of reducing costs whilst increasing output. Additional complications from outsourcing, nanotechnology and test methods are resulting in change, uncertainty and increased risks which need to be managed.
These are very interesting, exciting and challenging times in the world of pharmaceutical products, companies and regulatory bodies. There is a very valid argument that the pharmaceutical industry has some vital decisions to make regarding its future, particularly with about outsourcing production and the introduction of new technologies. Recent trends in outsourcing pharmaceutical production to Contract Manufacturing Organisations, Nanotechnology, introduction of Rapid Methods of Microbiology and the uncertainty of the ‘unknown’ means that life will be anything but quiet. It was Donald Rumsfeld who quoted “Reports that say something hasn’t happened are always interesting to me, because as we know there are known knowns; there are things we know we know. We also know there are known unknowns; that is to say we know there are some things we do not know. But there are also unknown unknowns–the ones we don’t know we don’t know”. Contamination and Cross Contamination complicated by the factors of Contract Manufacturing, Nanotechnology and developing methods of Microbiology take us into a new era where the ‘unknown unknowns’ are waiting to be known.
Contamination, cross-contamination and its control has long been one of the main challenges in pharmaceutical production as nothing else is a greater liability to the health and safety of patients. Contamination is when a substance has impurities in it that can cause harm to living things and is basically anything that can be harmful to the pharmaceutical process or product. Contamination is dirt in the wrong place and anything affecting the integrity of a substance. The World Health Organisation (WHO) have for Good Manufacturing Practice (GMP) defined contamination for pharmaceutical products as the undesired introduction of impurities of a chemical or microbial nature, or of foreign matter into or on to a starting material or intermediate during production , sampling, packaging, storage or transport, and cross-contamination as contamination of a starting material, intermediate product or finished product with another starting material or product during production.
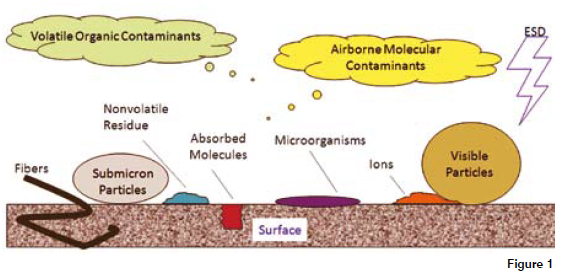
Contamination control requires an understanding of the microbial entry points and the various routes that can be taken to arrive where they are undesired. The diagram shows some of the sources of environmental contamination in addition to which there are cleaning agents, active Ingredients, decomposition products and materials, synthetic intermediaries, excipients, other residues, unfiltered air, room air, machines, ancillary equipment, containers, packaging, and the main source people. People are the problem as often they are the only real source of micro-organisms, generating particles just by being there. The routes of contamination need to be identified as these contaminated particles have to be controlled.
Contamination comes in the form of particles which is one of the smallest units of matter in the world. It is termed as a molecule (when it is made up of two or more non-metal atoms) or a compound (where two atoms are bonded together). One of the major reasons contamination and particles are difficult to comprehend is the fact they are so small and in the majority of cases invisible to the naked eye. Particles are defined as bodies with definite physical boundaries in all directions with diameters ranging from 0.001 microns to 100 microns (where a micron is equal to one millionth of a meter). To put this in perspective the measurement of 25mm is equal to 25,400 microns and the eye of a typical needle is equal to 749 microns. The ability to see individual particles depends upon the eye itself, the quality of light, the background and the type of particle. In ambient air 99 per cent of all airborne particles by count are less than 1 micron in size. The typical eye cannot see below 30 microns.
The pharmaceutical industry, like many others has now become embraced in the world of Nanotechnology. This is the ability to manipulate particles/matter at the atomic and molecular level to create materials with very varied and new properties. The term Nano comes from the Ancient Greeks meaning Dwarf and scientifically one Nanometer represents the size of one billionth of a meter. Again to try and picture this one Nanometer is 40,000 times smaller than the thickness of a human hair. This ability to work at such small scales promises much in such fields as drug delivery, gene therapy, research and clinical applications. In fact we have original drugs, generics, biosimilars and now nanosimilars!
Particles can be viable or non-viable and it is widely accepted that micro-organisms live and move around on 0.5 micron to 5 micron particles. The motion and movement of particles is affected by the velocity of the fluid for examle air; the inertia of the particle; gravitational, electrical, magnetic forces; the viscous drag and the collision with molecules, other particles or surfaces forming agglomerates. To put this in perspective and as an example five 1 micron particles colliding in the right conditions will form a five micron particle.
Particles are generated by everything and using vectors move about freely within our environments. Examples of vectors include air and other gases, water and other liquids, physical objects and of course people. People generate 80 per cent of particles; equipment only 15 per cent and the environment the remaining 5 per cent. In pharmaceutical manufacturing particles commonly found include:
Particulate matter proliferates in pharmaceutical manufacturing for a number of reasons. Manufacturing has so many stages with the product constantly in contact with process. There is process precipitation and the opportunity for chemical breakdown, aging and interaction with so many different materials. This leads to contamination and cross contamination from raw materials, containers, packaging, other materials, cleaning, storage, people and the environment.
Given all of this it is perhaps surprising that any pharmaceutical product gets to its destination, the patient, in the safe state it does. However, the foregoing establishes beyond doubt the risks to human health and safety that exist at every stage of manufacture and why a risk management policy in pharmaceutical facilities is so essential.
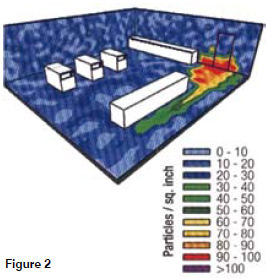
In todays safety conscious and highly regulated / legislated society the term risk management is surely familiar to every manager in every pharmaceutical organisation. An understanding of risk management and risk assessment is today becoming a pre-requisite for those working in quality control and quality assurance and those active in pharmaceutical and medical device production. Quality risk assessment is a mandatory requirement.
Risk management is defined as the forecasting and evaluation of financial risks together with the identification of procedures to avoid or minimise that impact (Oxford English Dictionary). The European Medicines Agency through its document ICH Q9 Quality Risk Management sets out in depth how Risk Management now plays such an important part in GMP to the extent that ICH9 was incorporated into Chapter 1 of the GMP Guide in July 2008.
All pharmaceutical products and processes have an associated risk which is why, and without exception, all the regulatory bodies now use a risk based approach. As early as 2002 the FDA announced their risk based approach and this has been followed by most, if not all, of the regulatory bodies worldwide. Risk can be good or bad but there is little doubt that the use of the word risk has negative undertones. This negativity extends to some future event that may or may not occur. There is a wealth of information and a whole professional industry associated with risk and its potential to become catastrophic and most of the experienced analysts would agree that damage to human health and the environment are at the centre of risk management.
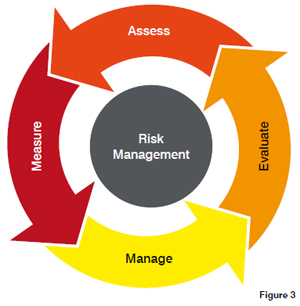
The risk management process often starts with a risk management plan which is drawn up by a risk management team. The team should represent as wide a view as possible of the various operational functions within the organisation. The process then moves through the following (not exhaustive) activities: Risk: Assessment; Identification; Analysis; Evaluation; Control; Reduction; Acceptance; Communication; and Review. It can be argued that perhaps the most important aspect of any risk management is the risk review. Risk management is a continuing process and one that should never end. One thing in life that is certain is change and that small word says everything about what is, or should be done, to cope with and address change within a risk management plan.
Should the following questions be asked?: How do you know that your risk management methods are working or not? What are the consequences if they do not work? It can be argued that the biggest single risk for any organisation is the risk that their risk management process doesn't work! This article does not propose to go into the plethora of models and tools that can be used within a Risk Management Process or Plan as such information is readily available from a number of sources and far more eminent authorities than the writer. It will instead look briefly into some of the risks associated with some of the challenges already highlighted.
The current financial pressures combined with many other reasons such as concentrating on core competencies are encouraging the pharmaceutical industry to look to contract manufacturing because of benefits such as cost savings through the economies of scale amongst others. With benefits comes risks and it is clear that no one organisation can afford a serious Health or Environment incident. Manufacturing new and different drugs in the same rooms, using the same and similar equipment for multiple end-users brings with it hazards especially as the drugs themselves get more and more potent. In addition to potential quality risks comes a lack of control, compromising Intellectual Property rights, language and cultural concerns/differences, on time delivery issues, potential shortage of supplies and competition for priority of supply etc. The parties in these relationships will have to address and amend their Risk Management to cater for this change to include using tools such as Risk-MaPPing to prevent cross-contamination from whatever source. Their Risk Management Plans will have to be continually monitored and amended.
Let's get back to the unknowns. Pharmaceutical nanotechnology has already provided some very great benefits in diagnosis and treatment of disease. On the negative and risk side it has raised social, ethical, scientific and not least regulatory issues. There are some unanswered questions on health risks many of which appear to be carcinogenic/highly toxic. There are also environmental concerns and problems and uncertainty about the general safety of the science. The risks are extremely complex but will include amongst others, the release of nanoparticles into the environment; political risks regarding the impact on economic development of countries; intellectual property and business risks. Any risk management plan or policy/process relating to nanotechnology will involve a complex risk assessment and identification process needing to be flexible, dynamic, and scientific.
Micro-organisms are responsible for much harm to the safety and integrity of pharmaceutical products. They are found by the millions in or on the environment, water, raw materials, containers and closures, equipment and not least people. Not all organisms are bad, but again the unknowns come into play as it is generally accepted by all in the industry that so little is known about them, how they communicate and just how many different species exist. To help protect the quality and integrity of pharmaceutical products we have testing methods which help us identify and deal with this contamination but as with all testing there are limitations. One of the major problems remains the time taken to identify if there is anything contaminating products and if so identifying them. Rapid Microbiological Methods are increasing in use as is Real Time Particle Counting but with them the uncertainty or risk that they have limitations and need development to provide even better solutions. Again Risk Management will include some testing and validation and again needs to be monitored, continuously improved and changed as the risks of this new technology are identified.

It has already been mentioned that people are the problem, which raises another complex issue of Risk Behaviour. The terms Contamination and Risk are raised every day and are mentioned constantly in pharmaceutical production. People being people invoke the human factors of familiarity and complacency. Historically where there has not been a problem why should there be one now. In order to be effective at reducing complacency and familiarity an understanding is needed how At risk behaviour occurs.
So is prevention better than cure? The rationale behind this saying is that it is better and cheaper to prevent problems before they occur. Familiarity breeds contempt becoming accustomed to familiar things that you no longer value them. Colin Powell wrote If it ain't broke don't fix it is the slogan of the complacent, the arrogant or the scared. It is an excuse for inaction, a call to non-arms.
If particles are kept out of the critical areas in a production unit there is less risk of contaminated products being produced.
Prevention costs a lot less than the cure, which is recalled product, waste, scrap, regulatory fines, suspension or ultimately closure.
Place contamination control flooring to prevent foot-borne, wheel-borne and air particles in gowning rooms, transfer air locks, warehousing to manufacturing.
References are available at www.pharmafocusasia.com
-- Issue 19 --