Pharmacovigilance is a complex process for which robust systems are essential. A strong PV system is an important part of the overall medicinal product Regulatory system. It reflects on the stringency and competence of the Regulatory bodies in regulating the market and ensuring the safety and effectiveness of the medicinal product.
Pharmacovigilance (PV) is principally concerned with the identification of Adverse Drug Reactions (ADRs) and reduction of the associated risks. Detection and reporting of ADRs can make prescription of medicinal products much safer and more effective. This is possible only if pharmaceutical companies and patients report the ADRs as and when they occur.
Before a medicinal product is marketed, its safety and efficacy exposure is limited to its use in clinical trials. Generally, clinical trials cover limited number of patients with strict inclusion criteria, often excluding special patient groups like those with co-morbid conditions, children, elderly and pregnant women. Hence, they do not reflect the experience in bigger population and in different geographical regions. People from different geographical regions differ from one another with respect to genetics, food habits, life style, clinical practices, etc. This makes it obligatory to maintain a constant vigil on the use of medicinal products during the post-marketing period.
PV is a major post-marketing tool to ensure the safety of a medicinal product. Apart from the respective drug regulating authorities in each country, International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, Pharmacovigilance Planning-ICH E2E and World Health Organization-Uppsala Monitoring Centre (WHO-UMC) also play key roles towards developing, enhancing and monitoring global PV system. A PV system is defined as a system used by an organisation to fulfil its legal tasks and responsibilities in relation to PV that monitors authorised medicinal products’ safety and detect if any change to riskbenefit balance.
After the thalidomide disaster in the year 1961, WHO worked along with its Collaborating Centre to establish a programme for International Drug Monitoring. Through this programme, WHO promoted PV at the country level. At the end of 2010, 134 countries were part of the WHO-PV Programme.
• Augment patient care and patient safety with respect to the use of medicinal products
• Run public health programmes by providing reliable information for the effective assessment of the risk-benefit profile of medicinal products.
A well-structured PV system can establish safety data in a precise manner from various levels of social healthcare environment. Building-up an effective system demands for harmonisation of different criteria, which requires an early, well thought of plan that ensures perfect execution and tangible benefits.
Pharmacovigilance is all about drug regulations and is based on thorough collaborative ties, coordination, communications, and public relations. The most suitable location for setting up a PV centre is dictated by the political governance and its healthcare priorities, including willingness to do, law enactment, its enforcement, funding, organisation, staffing, training, and development.
• A properly structured drug safety management team to intensify the communication among the PV network. This will assure an organised structure and smooth functioning. Meetings among the PV physicians, managers, and technical agencies need to be held from time to time
• A countrywide database which provides provision for collating and managing ADR reports
• A national PV advisory committee
• A clear approach, to be communicated in detail, in regular situations as well as situations of crisis
• Funding to run different grounds of a system.
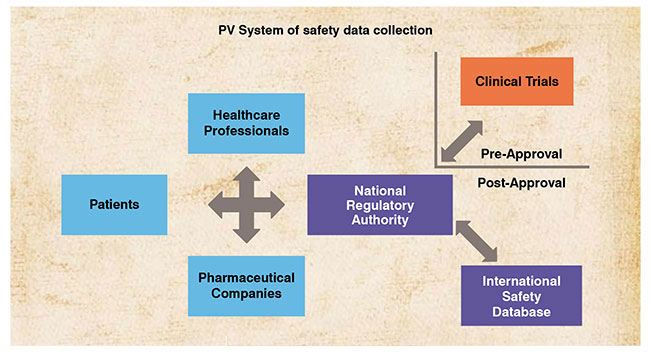
Developing guidelines and communications with the health authorities-a general guideline is a standard strategy to confirm that the PV system at all levels meets the national and international standards and regulations. Getting into regular communications with the health authorities, local, regional and national bodies, and professionals involved in clinical medicine, pharmacology, toxicology, epidemiology, briefing them about the importance of the project and its applicability in modern therapeutics.
• Should have adequate qualified and experienced man power to run the system - PV staff should have complete knowledge regarding data collection and verification, coding of drugs and adverse events, causality assessment, signal detection, risk management, interpreting the data obtained etc.
• Setting up of PV centres - Creating a database which is safely stored, retrievable and guarded by required degrees of confidentiality. Some of the basic technological requirements to be met by the PV centre are uninterrupted electric supply and ensuring that the intercom, multi connection telephone, computer, printer, fax, internet, photocopiers are in working condition. The PV centre should have adequate back up facilities so that work is not hampered during breakdowns; anticipated or sudden
• Continuous monitoring and improving the PV system performance - the capacity building processes include the management of the medicinal products, the system and the individual in the network, and effective monitoring of medicinal products from a safety perspective. The aim of capacity building is to create a robust system without creating changes in social structures, resources, technologies and personalities
• Data acquisition through ADR reporting form - preparing an ADR reporting template and to make it readily available, in different hospital settings and general practitioners, based on which they can provide relevant information to the PV centre
• Creating public awareness for ADR reporting – conducting workshops and meetings in the different healthcare institutions, academic institutions, promotional events to educate patients and healthcare professionals on the importance of reporting ADR through medical journals, professional publications, and seminars, and developing printed handouts. All these are done to notify healthcare professionals and public about the definitions, goals, scope, and methodology of the PV system to create awareness about its present relevance
• Detecting signals on the reported adverse drug events - based on the case reports, the PV centre should be able to detect a signal with regards to probable ADR
• Be associated with health authorities, pharmaceutical companies, other professional associations /organisations and WHO and its collaborating bodies so that information on observed adverse reactions is shared / notified time to time which may include cases with particular interest; and take proper preventive measures whenever those are necessary.
The PV system needs to deal with large population and the rate of reporting governs the estimation of the money needed to run the complete system. Huge investment is required in terms of collection of data from the actual source to transforming it into a Regulatory reportable format. Funding can be obtained from various parties, such as drug Regulatory authority, university departments, health insurance companies, and professional associations.
PV is a complex process for which robust systems are essential. A strong PV system is an important part of the overall medicinal product regulatory system. It reflects on the stringency and competence of the Regulatory bodies in regulating the market and ensuring the safety and effectiveness of the medicinal product. The foundation for building a robust PV system demands skilled manpower, support from healthcare professionals and pharmaceutical companies, safety awareness among the patients, information technology, and funding. The system needs to be refined with the help of PV experts in collaboration with technology. Establishing a robust PV system is a tough job; however, with thorough preparation, a practical approach, continuing zeal and motivation of the concerned staff, it can be achieved. PV ensures that future generations will not condemn the present one for its apathy, indifference, and callousness to the gravity of the situation. Without PV, modern medicine will be continued to be called as allopathy.
References:
1. Saha PS. Starting-Up A Pharmacovigilance Network from Scratch: A Brief Plan. JPRCP, 2014;4(1):58-66.
2. Jadav BH, Thula KC and Maheshwari DG. Regulatory requirements of pharmacovigilance system and its Comparison in India and USA. JGTPS. 2015;6(1):2351-56.
3. Nwokike J. Technical Assistance for the Establishment of a Pharmacovigilance and Medicine Safety System in Rwanda. Submitted to the US Agency for International Development by the Strengthening Pharmaceutical System (SPS) Program. Arlington, VA: Management Sciences for Health; 2009:1-26.
4. European medicines Agency. Guideline on good pharmacovigilance practices (GVP). Module 1 – Pharmacovigilance systems and their quality systems. JUN-2012 Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129132.pdf. Accessed on 07-AUG-2017.
5. Chakrabarty M, Thawani V. Starting a pharmacovigilance center: Actions for implementation. J PharmacolPharmacother. 2011;2(4):295-299.
6. World Health Organization and Global Fund. Minimum requirements for a functional pharmacovigilance system. Geneva: World Health Organization; 2010. Available at www.who.int/medicines/areas/quality.../PV_Minimum_Requirements_2010_2.pdf. Accessed on 07-AUG-2017.
7. WHO Pharmacovigilance Indicators: A Practical Manual for theassessment of pharmacovigilance systems; 2015. Available at apps.who.int/medicinedocs/documents/s21970en/s21970en.pdfAccessed on 07-AUG-2017.